Chemical Kinetics NEET Previous Year Question Paper: NEET PYQ (Past 10 Year)
The National Eligibility cum Entrance Test is a national-level entrance exam conducted by the NTA. It includes questions from Physics, Chemistry, and Biology. Chemistry is further divided into Physical, Organic, and Inorganic Chemistry. In the NEET exam, the Chemistry section has 45 questions out of the total of 180 questions. The unit “Chemical Kinetics” is a part of the Class 12 syllabus. Solving Chemical Kinetics NEET PYQ provides knowledge about the mechanisms and factors affecting reactions.
This Story also Contains
- Chemical Kinetics NEET PYQ Analysis (2020-2025)
- Chemical Kinetics NEET PYQ
- Important Topics of Chemical Kinetics
- Tips while Solving Chemical Kinetics NEET PYQ

Clearing the NEET exam requires a smart and strategic approach as the syllabus is vast. Chemical Kinetics is the branch of chemistry that deals with the rates of chemical reactions. It involves the theory concepts as well as the mathematical calculations. Regular practice of MCQs is one of the best NEET preparation tips to improve accuracy. NEET PYQ chemical kinetics covers concepts like reaction order, half-life calculations, and real-world applications.
Chemical Kinetics NEET PYQ Analysis (2020-2025)
Chemical Kinetics helps aspirants learn about the Arrhenius equation and the collision theory of reactions. NEET chemistry chapter-wise weightage shows Chemical Kinetics holds 5% weightage. It is an important unit of the NEET chemistry syllabus. Attempting NEET PYQ chemical kinetics helps students identify important topics. The table below shows the number of questions that appeared in the previous years.
Year | No. of Questions |
2025 | 2 |
2024 | 1 |
2023 | 2 |
2022 | 2 |
2021 | 2 |
2020 | 2 |
Chemical Kinetics NEET PYQ
Numerical-based questions from Chemical Kinetics appear in the NEET exam. To solve them in less time, an effective NEET preparation timetable is important. The timetable should include 2-3 hours daily for solving the NEET question paper. Provided below are a few NEET chemical kinetics previous year questions with solutions. Going through them makes students familiar with the question pattern.
Question 1: If the half-life $\left(\mathrm{t}_{1 / 2}\right)$ for a first-order reaction is 1 minute, then the time required for $99.9 \%$ completion of the reaction is closest to: (NEET 2025)
Options
10 minutes
2 minutes
4 minutes
5 minutes
Answer: For a first-order reaction-
$t=\frac{6.9}{k}$ for $\sim 99.9 \%$ completion (more accurately, $t=\frac{6.91}{k}$ )
Also half-life for a first-order is given by
$t_{1 / 2}=\frac{0.693}{k} \Rightarrow k=\frac{0.693}{1}=0.693$
$\Rightarrow t=\frac{6.91}{0.693} \approx 10 \mathrm{~min}$$
Hence, the correct answer is option 1) 10 minutes.
Question 2: If the rate constant of a reaction is $0.03 \mathrm{~s}^{-1}$. how much time does it take for $7.2 \mathrm{~mol} \mathrm{~L}^{-1}$ concentration of the reactant to get reduced to $0.9 \mathrm{~mol} \mathrm{L}^{-1}$ ? (Given: $\log 2=0.3$) (NEET 2025)
Options
21.0 s
69.3 s
23.1 s
210 s
Answer: Given that
Initial concentration $[R]_0 = 7.2 \ \mathrm{mol \, L^{-1}}$
Final concentration $[R] = 0.9 \ \mathrm{mol \, L^{-1}}$
Rate constant $k = 0.03 \ \mathrm{s^{-1}}$
Given $\log 2 = 0.3$
Since the units of $k$ are $\mathrm{s^{-1}}$, this is a first-order reaction.
Formula: $t = \frac{2.303}{k} \log \left( \frac{[R]_0}{[R]} \right)$
Substitute: $t = \frac{2.303}{0.03} \log \left( \frac{7.2}{0.9} \right)$
This becomes $t = \frac{2.303}{0.03} \log(8)$
Now, $\log(8) = \log(2^3) = 3 \log 2 = 3 \times 0.3 = 0.9$
So, $t = \frac{2.303}{0.03} \times 0.9 = 76.77 \times 0.9 = \mathbf{69.093 \ \mathrm{s}}$
Hence, the correct answer is option 2) 69.3 s.
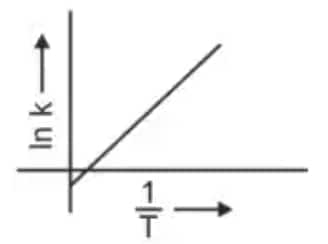
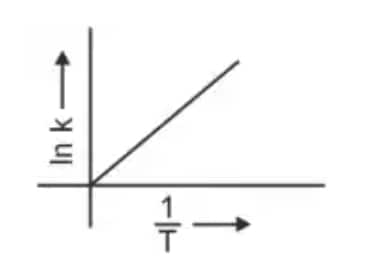
Question 3: Which plot of In k vs 1/T is consistent with the Arrhenius equation? (NEET 2024)
Options
3. 
4. 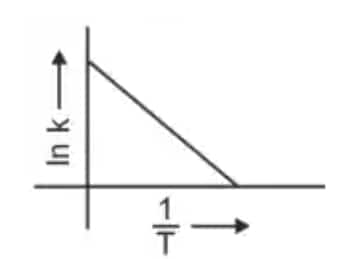
Answer: The Arrhenius equation is given as
$k = A e^{-\frac{E_a}{RT}}$
In $k$ vs $\frac{1}{T}$ gives a straight-line graph with slope $= -\frac{E_a}{R}$ and intercept $= \ln A$.

Hence, the correct answer is option (4) 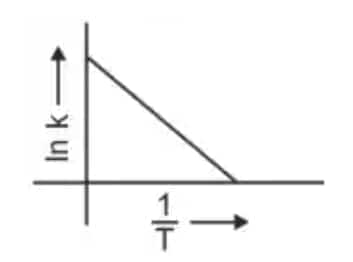
Question 4: For a certain reaction, the rate $R = k[A]^2[B]$, when the initial concentration of A is tripled, keeping the concentration of B constant, the initial rate would be: (NEET 2023)
Options
decrease by a factor of nine.
increase by a factor of six.
increase by a factor of nine
increase by a factor of three
Answer: $R = k[A]^2[B]$
$r \propto [A]^2$, if $[B]$ remains constant
$r_f \propto [3A]^2$
$r_f \propto 9 \times [A]^2 \propto 9 \times r_i$
So, the rate will increase 9 times the initial rate.
Hence, the correct answer is option (3), increase by a factor of nine.
Question 5: Given below are two statements: one is labeled as Assertion A and the other is labeled as Reason R :
Assertion A: A reaction can have zero activation energy.
Reasons R: The minimum extra amount of energy absorbed by reactant molecules so that their energy becomes equal to the threshold value is called activation energy.
In light of the above statements, choose the correct answer from the options given below: (NEET 2023)
Options
Both A and R are true, and R is the correct explanation of A.
Both A and R are true, and R is NOT the correct explanation of A.
A is true, but R is false.
A is false, but R is true.
Answer: The free radical reactions can have zero activation energy. In the free radical combination reaction in termination, steps have $E_a = 0$.
Hence, the correct answer is option (2), Both A and R are true, and R is NOT the correct explanation of A.
Question 6: For a first-order reaction $\mathrm{A} \rightarrow$ Products, the initial concentration of A is 0.1 M, which becomes 0.001 M after 5 minutes. Rate constant for the reaction in $\mathrm{min}^{-1}$ is: (NEET 2022)
Options
0.9212
0.4606
0.2303
1.3828
Answer: For a first-order reaction, the integrated rate law expression is
$\begin{aligned} & \ln \left(\frac{\mathrm{A}_0}{\mathrm{~A}_{\mathrm{t}}}\right)=\mathrm{Kt} \\ & \Rightarrow \quad \ln \left(\frac{0.1}{0.001}\right)=\mathrm{K}(5) \\ & \Rightarrow \mathrm{K}=\frac{\ln 100}{5}=\frac{4.606}{5}=0.9212 \mathrm{~min}^{-1}\end{aligned}$
Hence, the correct answer is option (1), 0.9212.
Question 7: The given graph is a representation of the kinetics of a reaction
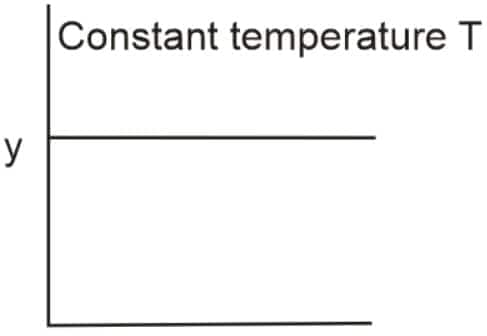
The y and x axes for zero and first-order reactions, respectively, are: (NEET 2022)
Options
zero order (y= concentration and x= time), first order (y= rate constant and x= concentration)
zero order ( $\mathrm{y}=$ rate and $\mathrm{x}=$ concentration $)$, first order $\left(\mathrm{y}=t_{1 / 2}\right.$ and $\mathrm{x}=$ concenration $)$
zero order ( $y=$ rate and $x=$ concentration), first order ( $y=$ rate and $x=t_{1 / 2}$ )
zero order ( $y=$ concentration and $x=$ time $)$, first order $\left(y=t_{1 / 2}\right.$, and $x=$ concentration $)$
Answer: For a zero-order reaction:
Rate $\propto [\mathrm{A}]^0$
$\Rightarrow \text{Rate} = k = \text{constant}$
$\Rightarrow$ Rate is independent of concentration.
For a first-order reaction:
$t_{1/2} = \frac{\ln 2}{k}$
$\Rightarrow$ Half-life is independent of concentration.
Hence, the correct answer is option (2), zero order ( $\mathrm{y}=$ rate and $\mathrm{x}=$ concentration $)$, first order $\left(\mathrm{y}=t_{1 / 2}\right.$ and $\mathrm{x}=$ concenration $)$.
Question 8: The slope of the Arrhenius Plot $\ln k \, v/s \; \frac{1}{T}$ the first-order reaction is $-5 \times 10^{3} \, K$. The value of $E_a$ of the reaction is. Choose the correct option for your answer. (NEET 2021)
[Given $R = 8.314 \ \mathrm{J \, K^{-1} \, mol^{-1}}$]
Options:
1. \( 41.5 \ \text{kJ mol}^{-1} \)
2. \( 83.0 \ \text{kJ mol}^{-1} \)
3. \( 166 \ \text{kJ mol}^{-1} \)
4. \( -83 \ \text{kJ mol}^{-1} \)
Answer: According to the Arrhenius equation
\( k = Ae^{-E_a/RT} \)
\( \ln k = \ln A + \ln e^{-E_a/RT} \)
\( \ln k = \ln A - \frac{E_a}{R} \left( \frac{1}{T} \right) \)
The slope \( \left( \ln k \ \text{vs} \ \frac{1}{T} \right) \) of the above equation
\( m = -\frac{E_a}{R} \)
Given, \( m = -5 \times 10^3 \ \text{K} \) and \( R = 8.314 \ \text{J K}^{-1} \text{mol}^{-1} \)
\( -5 \times 10^3 = -\frac{E_a}{8.314} \)
\( E_a = 5 \times 10^3 \times 8.314 \ \text{J/mol} \)
\( E_a = 41.57 \times 10^3 \ \text{J/mol} \)
\( E_a = 41.5 \ \mathrm{kJ \ mol^{-1}} \)
Hence, the correct answer is option (1), \( 41.5 \ \text{kJ mol}^{-1} \).
Question 9: An increase in the concentration of the reactants of a reaction leads to a change in: (NEET 2020)
Options
Collision frequency
Activation energy
heat of reaction
threshold energy
Answer: An increase in the concentration of the reactants leads to an increase in the collision frequency. Threshold energy, activation energy, and heat of reaction are not affected by the increase in concentration. This is because, due to the increase in concentration, the number of particles increases and hence the number of collisions.
Hence, the correct answer is option (1), Collision frequency.
Question 10: The rate constant for a first-order reaction is $4.606 \times 10^{-3} \mathrm{~s}^{-1}$. The time required to reduce $2 g$ of the reactant to $0.2 g$ is: (NEET 2020)
Options
1000s
100s
200s
500s
Answer: We are given:
$K = 4.606 \times 10^{-3} \ \mathrm{s}^{-1}$
Thus, the reaction is of the first order.
We have:
$K t = \ln \frac{A_0}{A_t}$
$4.606 \times 10^{-3} \ t = 2.303 \ \log \left( \frac{2}{0.2} \right)$
Thus, $t = 0.5 \times 10^3 \ \mathrm{s}$
$t = 500 \ \mathrm{s}$
Hence, the correct answer is option (4), 500s.
Important Topics of Chemical Kinetics
Chemical Kinetics is a scoring unit in chemistry. It is among the do-or-die chapters for NEET. Questions in the NEET 2024 question paper come from the Chemical Kinetics unit. Chemical kinetics NEET PYQ includes questions on how reactions occur and the different factors affecting them. Students thinking about how to study chemistry for NEET should start with this unit. Given below are a few important topics from this unit.
Topics | Description |
Defined as the change in concentration of reactants or products per unit time. | |
Order of Reaction | For complex reactions, the order is determined by the rate-determining step. |
The number of reacting species involved in an elementary reaction. | |
Rate Constant (k) | A proportionality constant that relates the rate of the reaction to the concentrations of the reactants. |
Activation Energy (Eₐ) | The minimum energy required for a reaction to occur. |
This equation relates the rate constant to temperature and activation energy. It is important for understanding how temperature affects reaction rates. | |
Half-Life (t₁/₂) | The time required for half of the reactant to be consumed is known as half half-life. |
Factors Affecting Reaction Rates | Understanding how concentration, temperature, and catalysts influence reaction rates is important. |
Tips while Solving Chemical Kinetics NEET PYQ
Solving previous year questions regularly builds confidence. It helps students understand the types of questions asked in the exam. Aspirants can start by solving the 20 important NEET chemistry questions to make their preparation strong. Given below are a few tips that they can use to solve NEET chemical kinetics previous year questions.
Students have to understand how to obtain rate laws from the rate-determining step. Accessing the NEET chemistry mock test improves score and speed.
Learn how to determine the order of a reaction from experimental data and not just by stoichiometry.
Get familiar with the rate equations for zero, first, and second-order reactions. Study only from the Chemistry books for NEET to avoid confusion.
Understand the role of activation energy and how it plays a part in the rate determination. Try to apply the Arrhenius equation to the temperature changes.
Solve NEET PYQ chemical kinetics by using the NEET chemistry question paper.
Prepare flashcards of important formulas for NEET chemistry. This helps to answer NEET chemical kinetics previous year questions confidently.
Popular Courses and Specializations
Browse Medicine Colleges by State
Questions related to NEET
On Question asked by student community
yes you can check , this will show as related to best college as per your rank
Hello there,
With a NEET UG 2024 score of 504 and belonging to the OBC category, you have a fair chance of getting admission into self-financed medical colleges, especially in states where the cutoffs tend to be slightly lower. Admission chances depend on factors like the specific state, the number
Hello Koushisk,
Yes, you can absolutely attend NEET UG 2025 as an engineering student. There are no restrictions on appearing for NEET UG based on your current field of study. Since you've completed your 12th grade with the required subjects (Physics, Chemistry, Biology, and English), you are eligible to reappear
Sorry aspirant, the information you provided is unclear and difficult to understand. Could you please clarify the following:
- Paramedical without NEET cutoff: Are you referring to a paramedical course that does not require the National Eligibility cum Entrance Test (NEET) for admission?
- 172 MBC community Villupuram government medical College la
Hello student,
I understand that you have a paramedical course without NEET cutoff and obtained a score of 172.
However, I'd like to clarify a few things to provide the most accurate guidance:
- Specific Course: Could you please specify the name of the paramedical course you're referring to? This will